The importance of bonds
Without “bonds” there wouldn’t be a great deal of chemistry to find out about. Chemical reactions are essentially changes to the way in which atoms and ions are bonded together. Physical processes like boiling, melting and dissolving all involve bonds. Crystals are held together by bonds, water forms drops because of bonds. Developing a good understanding of bonding is obviously going to be essential for further progress in chemistry. So, what actually are “bonds”?
Defining bonds
In chemistry, bonds are the attractive forces between atoms, ions and molecules that cause them to form specific structures. There is more than one way in which a bond can form. Straight after GCSE students tend to divide bonds into three basic types – covalent, ionic and metallic, and, while partially correct, this is something of a simplification. For a start, we should consider intermolecular forces as bonds too – they are still attractive forces albeit much weaker than the three basic types mentioned previously. In addition, there is not always a sharp distinction between one bond type and another; often the situation is more like a spectrum where you can find examples that gradually shift from one type of bond to another. At A-level, you will also need to recognise when specific variations of each bond type can occur, (for example dative bonds, ligand-metal bond and ion-dipole bonds etc). All of these types will be described in subsequent sections.
Electrostatic forces
However, regardless of the particular type of bond in question, all bonds are essentially the same thing – overall electrostatic attractions. This means that bonds are the attractive forces between one thing with positive charge and something else with a negative charge. Remember: two particles with opposite charges will attract one another, with a force acting on each one to draw them together. In contrast, two particles with the same charge will repel, each particle experiencing a force pushing it away from the other.
Below is a quick summary of the directions of the electrostatic forces between two particles for different combinations of charge. I will produce a more detailed article about electrostatics in another section.
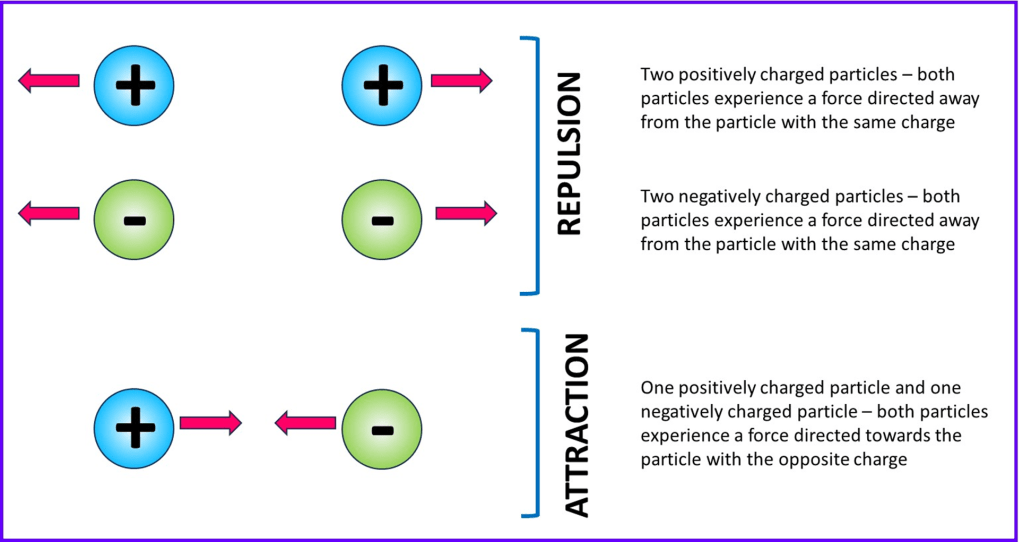
Bonds are somewhat more complicated than the simplified pairs of particles shown in the diagram above, because there are usually more than two charged particles present at once meaning that multiple electrostatic forces occur together. Sometimes both repulsive and attractive forces are present simultaneously, but bonds are overall net attractions – the attractive forces exceed all the repulsions. If this wasn’t true, the bond wouldn’t exist.
Types of bonds
Exactly what the positive and negative particles are varies from one type of bond to another (though of course ultimately, the positive charge can be traced back to protons somewhere and the negative charge to electrons, as these are the source of all charges in chemistry).
Here are the identity of the positive and negative species in the common types of bonds familiar to students at the start of A-level:
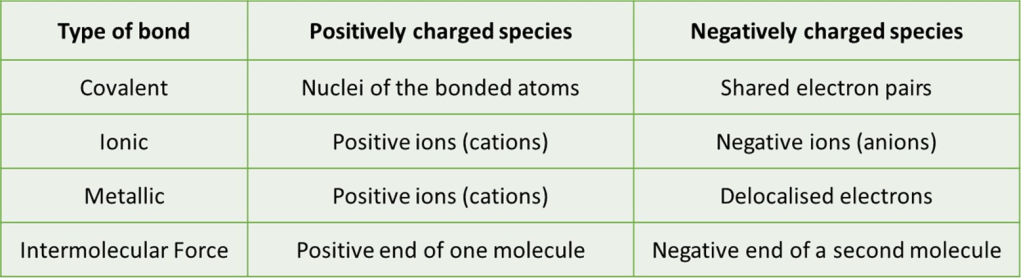
Later sections will look at how these attractions arise in more detail and consider how you can tell which type of bond is present in different circumstances.
Breaking bonds
There is a very common and significant misconception that students have about bonds. I am not entirely sure why, but students very often think that breaking bonds releases energy. Unfortunately, this is completely wrong!
“Breaking bonds always requires / uses / needs energy. And the reason for this is that bonds are an attraction.“
I think that comparing the situation with two very strong magnets can be quite helpful. Each magnet could represent one atom bonded to another. Now imagine trying to separate the magnets if they were stuck together. It would be quite hard work to overcome their mutual attraction and you’d end up having to use a lot of your energy to suddenly snap them apart and keep them separated:
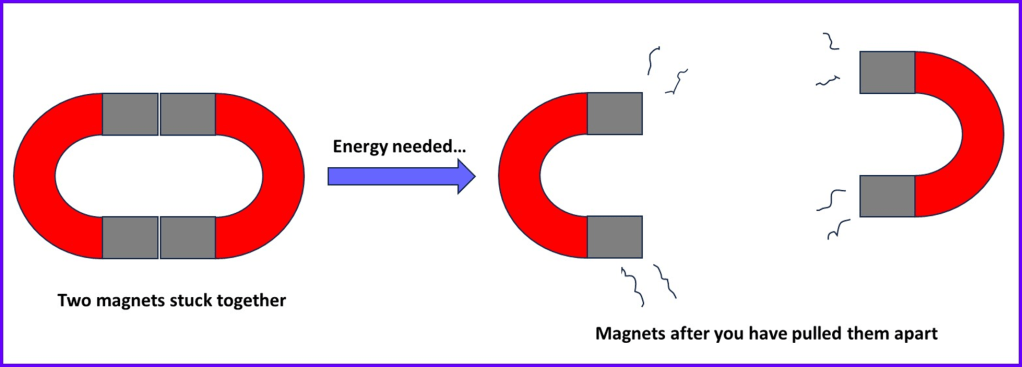
If two atoms are bonded together then they are attracting one another, with forces acting to pull them together. Like with the magnets, you would have to exert some effort to separate them. So it is important to remember that bond breaking requires energy and this will be extremely important in many areas of chemistry .
**There are different types of forces between protons and neutrons in a nucleus, but this is not something that is considered part of chemistry. These forces are not electrostatic, so we won’t consider them any further here, but it’s an interesting area to investigate.
“Copyright Simon Colebrooke 30th August 2025.”
Update History
 chemistryexplained.uk
chemistryexplained.uk