Check your answers and understanding by reading through this Answer Set.
1. A table of data reports that a copper atom has an atomic radius of 140 pm.
a). What is the diameter of the copper atom in pm?
The diameter is double the radius, so 2 x 140 = 280 pm.
b). What is the diameter of the copper atom in nm?
1 pm = 10-12 m and 1 nm = 10-9 m.
This means that 1 nm = 1000 pm.
Hence, to convert the diameter from pm into nm we divide by 1000.
Diameter of a copper atom = (280 / 1000) nm = 0.28 nm.
c). What is the diameter of the copper atom in m?
Since 1 m = 1012 pm, to convert the diameter from pm to m we divide by 1012.
Diameter in m = (280 / 1012) = 2.80 x 10-10 m.
d). The thin edge of a coin is 1.85 mm in thickness. Assuming it is entirely made from copper, how many atoms lined up end-to-end would fit across the coin edge?
Since we now have the diameter of a copper atom in m it makes sense to convert the dimension of the coin into m also:
1 m = 1000 mm, so we need to divide the 1.85 mm by 1000 to convert to m.
This gives coin thickness = 1.85 x 10-3 m.
Finally, we can calculate how many atoms fit across the edge of the coin by dividing the thickness by the diameter of each atom.
Hence, number of atoms = (1.85 x 10-3) / (2.80 x 10-10)
Number of atoms = 6, 607, 143
This is over six and a half million atoms!
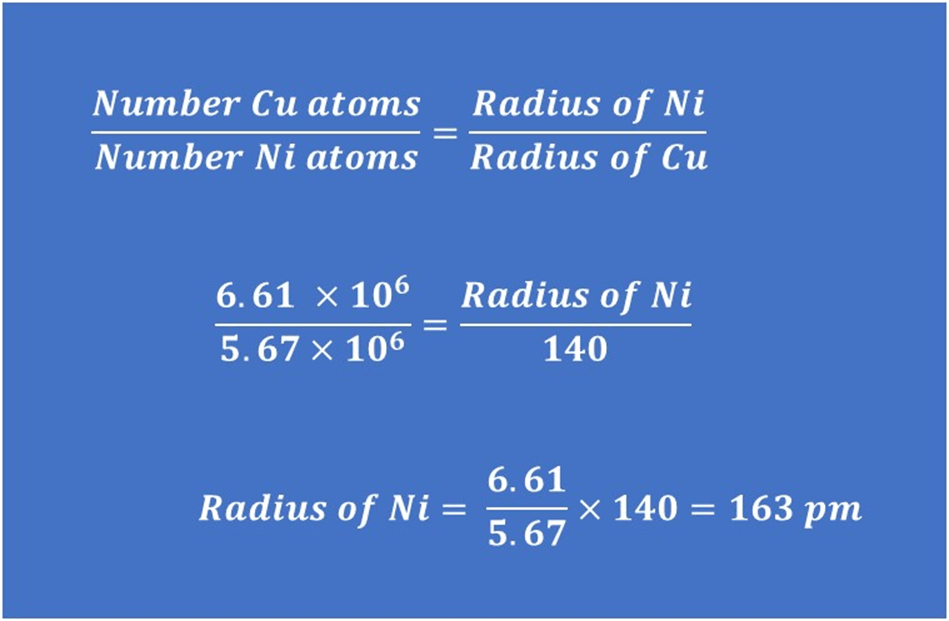
e). If 5.67 x 106 nickel atoms would fit across the same coin edge. What is their radius in pm?
There are two ways of answering this question:
i) A similar process to the question above, starting with dividing the coin thickness by 5.67 x 106 to get the diameter of one nickel atom in m. This can then be converted to pm by multiplying by 1012 and finally, dividing the diameter by 2 to get the radius. This gives a value of 163 pm.
ii) Find the ratio of the number of copper atoms to the number of nickel atoms – this must be the same as the ratio of the nickel radius to the copper radius (since a larger diameter would require fewer atoms to cover the same distance). The working is shown below, I have rounded the answer to part d) and used the value of the copper radius in pm given at the start of the question.
2. An aluminium needle has a thickness equivalent to
2.72 x 106 aluminium atoms laid end-to-end. What is the thickness of the needle in metres if an aluminium atom has a radius of 0.184 nm?
3. A potassium atom has radius of 2.75 Å, whereas a selenium atom has a radius of 0.190 nm – which is larger?
4. a. A gold atom has an atomic radius of 166 pm. Convert this distance into m.
b. Using the value in 4.a. calculate the volume of the gold atom in m3 if the volume of a sphere is given by this equation:
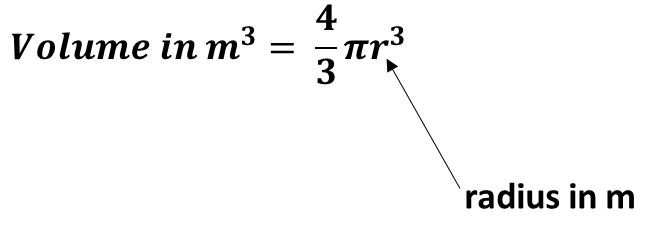
c. If atoms are imagined as spheres, it is not possible to stack lots of them together without any space being left in between them. Only 74% of the volume of an object can be occupied by atoms.
Calculate how many atoms must be in a flake of gold foil if the foil is 1 cm2 and has a thickness of 0.0001 m.
5. a. A small copper coin has a mass of 3.56 g. If there are
3.37 x 1022 copper atoms in the coin, what is the mass of one copper atom in g?
b. Copper atoms have a relative atomic mass of 63.5 whereas iron atoms have a relative atomic mass of 55.8. How many iron atoms would be needed to give a coin of the same overall mass?
“Copyright Simon Colebrooke 26th March 2025”
 chemistryexplained.uk
chemistryexplained.uk