The previous sections described the method for preparing a standard solution with known concentration. Key to that were three principles:
A). Accurately measuring the amount of solid solute used to make the solution.
B). Ensuring that all of the solute ends up in the volumetric flask.
C). Knowing exactly what volume of solution is prepared.
A careful method was outlined that enables these three objectives to be achieved. The concentration could be calculated using the mass of solid dissolved and the volume of solution prepared. However, students often make mistakes when carrying out the method and this affects whether the solution that is actually prepared has the calculated concentration or not.
Common method errors when making a standard solution
Here are some common errors and the effect they have:
1. The solid is not “weighed by difference”. The student zeros the digital balance with a weighing boat on top, adds the calculated mass of solid and then tips that into a beaker, before continuing with the method.
Why is this an error? How does the solution concentration compare to the calculated concentration?
Some small pieces of solid will be left on the weighing boat and not transferred into the solution. The mass of solid actually used is less than the calculated amount. The solution concentration is less than calculated.
2. The solid solute is not dissolved in the solvent in a beaker before being transferred to the volumetric flask. The student then has trouble getting all the solid to dissolve, so warms the volumetric flask on a hot plate to speed the process up.
Why is this an error? How does the solution concentration compare to the calculated concentration?
Volumetric flasks are not designed for heating. They are calibrated to a particular volume at a set temperature, often 20 OC. Heating the flask can change the volume required to fill it to the graduation line; for example the flask might expand, and subsequently hold a larger volume. It is possible that heating might even alter the shape of the flask permanently. If the shape of the flask changes, we will no longer know what volume it holds and so can never know the concentration of the solution; it could be more or less concentrated than calculated.
3. After dissolving the solid in a beaker and using a glass rod to stir the solution, the student removes the glass rod and places it on the lab bench.
Why is this an error? How does the solution concentration compare to the calculated concentration?
Without rinsing with distilled water back into the beaker, droplets of solution will be left on the end of the glass rod. These will contain some of the dissolved solute and this will end up on the lab bench and not in the volumetric solution.
The actual solution concentration will be less than the calculated concentration.
4. The beaker / funnel are not rinsed into the volumetric flask.
Why is this an error? How does the solution concentration compare to the calculated concentration?
Without rinsing these pieces of equipment with distilled water and transferring that into the volumetric flask, some of the dissolved solute has been left in the used glassware.
The actual solution concentration will be less than the calculated concentration.
The next two errors are illustrated in the diagram below:
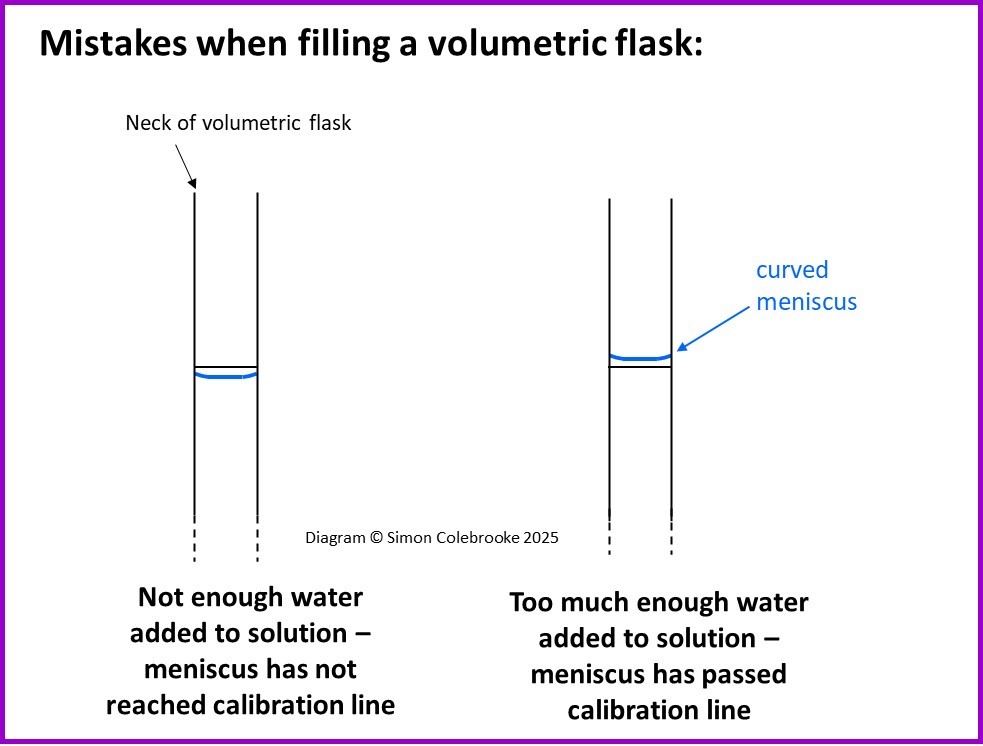
5. When filling the volumetric flask with distilled water, the meniscus is below the graduation line.
Why is this an error? How does the solution concentration compare to the calculated concentration?
The volume of solution at the end is less than the intended volume. The solute is in a smaller volume than it ought to be.
The actual solution concentration is greater than the calculated concentration.
6. When filling the volumetric flask with distilled water, the meniscus is above the graduation line.
Why is this an error? How does the solution concentration compare to the calculated concentration?
The volume of solution at the end is more than the intended volume. The solute is in a larger volume than it ought to be.
The actual solution concentration is less than the calculated concentration.
7. When filling the volumetric flask with distilled water the student over-fills the flask so that the meniscus is above the graduation line. To correct this, the student uses a dropping pipette to remove a little solution so that the meniscus is on the line correctly.
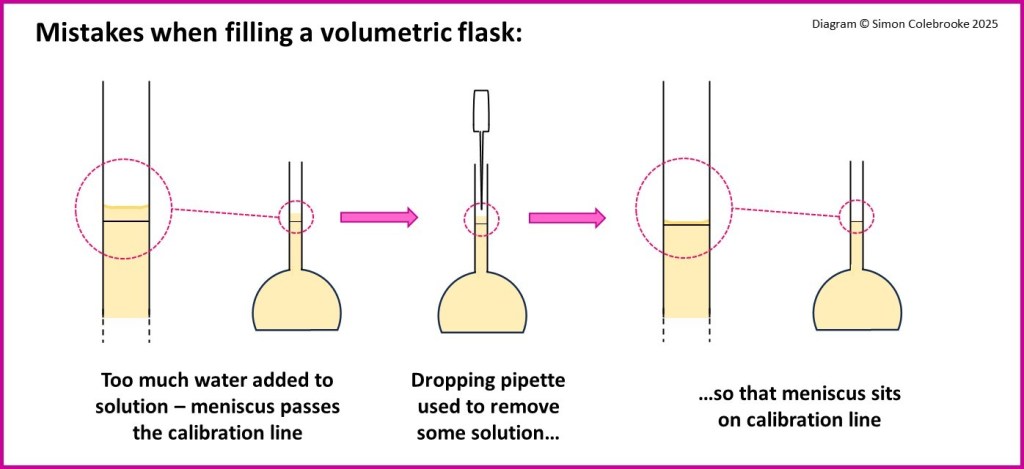
Why is this an error? How does the solution concentration compare to the calculated concentration?
When a small amount of solution is drawn into the pipette some of the solute is present. Although the solution will have the desired volume, some of the solute is missing.
The actual solution concentration is less than the calculated concentration.
8. The volumetric flask is not inverted after filling to the graduation line.
Why is this an error? How does the solution concentration compare to the calculated concentration?
The concentration and density of the portion of the solution that came from the beaker at the start of the method will be greater than that of the distilled water added later in the procedure. Without inverting the flask several times these two portions will only mix very slowly.
The solution concentration will vary at different locations in the flask; most of the solute will be near the bottom, so the concentration will be higher. The solution concentration will be either greater or lower than the calculated concentration at each location, but it will be unknown at all concentrations.
“Copyright Simon Colebrooke 15th November 2025″
Update History
 chemistryexplained.uk
chemistryexplained.uk