As you study chemistry at A-level it is important to appreciate the miniscule size of atoms and constantly remind yourself of this. It is also important to be able to convert between different units such as length and mass. The aim of this set of questions is to help with both these aspects.
You can imagine atoms as solid spheres for the purpose of answering these questions.
1. A table of data reports that a copper atom has an atomic radius of 140 pm.
a). What is the diameter of the copper atom in pm?
b). What is the diameter of the copper atom in nm?
c). What is the diameter of the copper atom in m?
d). The thin edge of a coin is 1.85 mm in thickness. Assuming it is entirely made from copper, how many atoms lined up end-to-end would fit across the coin edge?
e). If 5.67 x 106 nickel atoms would fit across the same coin edge. What is their radius in pm?
2. An aluminium needle has a thickness equivalent to
2.72 x 106 aluminium atoms laid end-to-end. What is the thickness of the needle in metres if an aluminium atom has a radius of 0.184 nm?
3. A potassium atom has radius of 2.75 Å, whereas a selenium atom has a radius of 0.190 nm – which is larger?
4. a. A gold atom has an atomic radius of 166 pm. Convert this distance into m.
b. Using the value in 4.a. calculate the volume of the gold atom in m3 if the volume of a sphere is given by this equation:
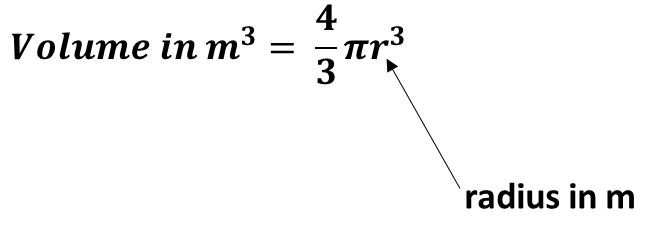
c. If atoms are imagined as spheres, it is not possible to stack lots of them together without any space being left in between them. Only 74% of the volume of an object can be occupied by atoms.
Calculate how many atoms must be in a flake of gold foil if the foil is 1 cm2 and has a thickness of 0.0001 m.
5. a. A small copper coin has a mass of 3.56 g. If there are
3.37 x 1022 copper atoms in the coin, what is the mass of one copper atom in g?
b. Copper atoms have a relative atomic mass of 63.5 whereas iron atoms have a relative atomic mass of 55.8. How many iron atoms would be needed to give a coin of the same overall mass?
Once you’ve answered the questions, click on the “A” icon to view the answers and check you are correct. Alternatively return to the current set of Notes with the “N” icon.
“Copyright Simon Colebrooke 26th March 2025”
Update History
Error in Q1 involving number of Nickel atoms, plus question numbering corrected 7th April 2025.
 chemistryexplained.uk
chemistryexplained.uk