Having read notes section 1.7 you should complete the questions below to make sure that your understanding is secure; the ideas will be continuously used in other areas of chemistry, so getting them correct will have to become second nature.
1. Work out the number of protons, neutrons and electrons in each of these atoms:
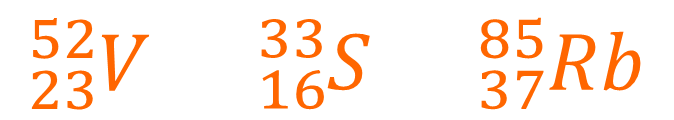
2. Write the nuclear symbol for the atom which has double the amount of protons and double the number of neutrons compared to the vandium atom (V) in Q1.
3. Work out the number of protons, neutrons and electrons in each of these positive ions:

4. Work out the number of protons, neutrons and electrons in each of these negative ions:

5. a. Give the nuclear symbols for species A-F which are composed of the following numbers of protons, neutrons and electrons:
| Species | Protons | Neutrons | Electrons |
| A | 26 | 30 | 26 |
| B | 17 | 20 | 18 |
| C | 1 | 1 | 0 |
| D | 26 | 32 | 26 |
| E | 25 | 31 | 23 |
| F | 33 | 40 | 36 |
b. Which of the species A-F above are atoms?
c. Which of the species A-F above are positive ions?
d. Which of the species A-F above are negative ions?
e. Which of the species A-F above are isotopes of one another?
6. Give the number of protons, neutrons and electrons in a atom of phosphorus-30.
7. Which of the species in the table below corresponds to an atom of calcium-41?
| Species | Protons | Neutrons | Electrons |
| G | 41 | 20 | 41 |
| H | 21 | 20 | 21 |
| I | 20 | 21 | 20 |
8. Write down the nuclear symbol of the isotope of the copper atom below which contains two more neutrons.
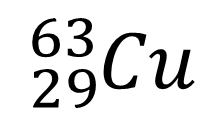
9. Which of the species in the table below is an isotope of fluorine-19?
| Species | Protons | Neutrons | Electrons |
| J | 9 | 11 | 9 |
| K | 19 | 20 | 19 |
| L | 9 | 10 | 10 |
| M | 10 | 9 | 10 |
| N | 9 | 10 | 9 |
10. a. Use the values given in the table below to calculate the percentage of the mass of a lithium-7 atom that is due to the electrons.
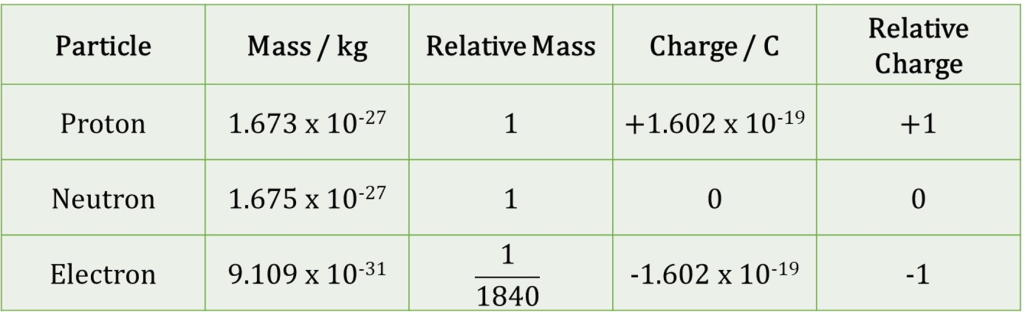
b. Suggest the nuclear symbol of the atom that will have the highest percentage of it’s mass due to electrons.
Once you’ve answered the questions, click on this link to view the answers and check you are correct:
“Copyright Simon Colebrooke 2nd April 2025”
 chemistryexplained.uk
chemistryexplained.uk