In this section you can read about a common procedure for preparing a solution in the lab. The method can be applied to all kinds of different substances and solvents – the principles are the same.
What are standard solutions?
Standard solutions are solutions with accurately known concentration.
Often, the standard solution is made by dissolving a known mass of a solid solute in water. The procedure followed is carefully designed so that no solute is lost during the process and high precision glassware (a volumetric flask) is used to measure the final volume of solution prepared. The procedure described below is very frequently used and can be applied to almost any solid substance and solvents other than water.
Procedure – key ideas
To be successful in preparing a standard solution it is essential that you do all three of the following:
A). Accurately measure how much solid solute is used to make the solution.
B). Make sure all of that solute ends up in the volumetric flask.
C). Know exactly what volume of solution is prepared.
The procedure below allows these three points to be achieved:
Step-by-step procedure
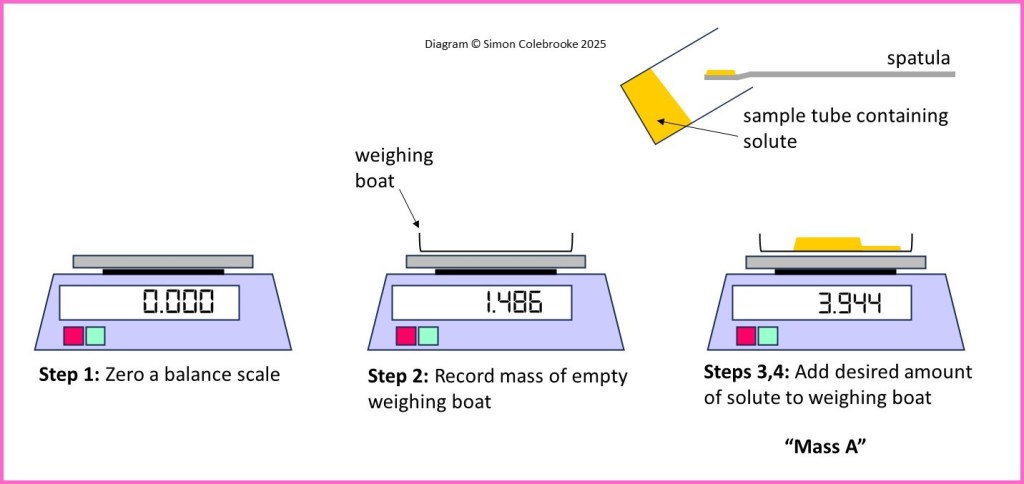
1. Zero the scale on a digital balance.
2. Add a weighing boat to the balance and take note of the reading. However, you will not need this number to calculate the concentration.
3. Use a spatula to add solute to the weighing boat (still on the balance) until you have added the required amount. This should have been determined in advance via a calculation and you can add this desired amount to the mass shown on the balance scale.
4. Record the mass shown on the balance scale, “mass A”.
5. Tip the solute from the weighing boat into an empty beaker (a 250cm3 beaker is usually suitable).
6. Record the mass of the weighing boat on the balance scale again, “mass B”.
7. Calculate the amount of solute added to the beaker by finding the difference between “mass A” and “mass B”.
WHY?
It is very likely that small pieces of solid will be left on the weighing boat. Weighing by difference means you know exactly how much solid was actually added to the beaker.
Often found students want to weigh the weighing boat with the solid on and then subtract the mass of an empty weighing boat, which they measured in advance. However, this method misses the small amount of solid left behind means you will think you have added more solid than you actually have.
8. Add some distilled water to the solute in the beaker. The exact amount does not matter, (so you do not need to measure it accurately), but it should be significantly less than the volume of the solution you are aiming to prepare.

WHY?
Later in the procedure you will transfer the solution from the beaker to a volumetric flask. You will need to rinse the beaker and any other pieces of equipment that have been in contact with your solution into the volumetric flask, so that none of your material is lost. You will need to use quite a lot of distilled water to do this, so must allow quite a bit of space in the flask for the washings.
9. Stir the contents of the beaker with a glass rod, until the solid has completely dissolved (you will then be left with a clear / see-through solution). If necessary, use the glass rod to crush up any solid pieces that don’t dissolve. You can also warm the beaker on a hot plate if the solid doesn’t dissolve at room temperature.
WHY?
It is not easy to get a solid to dissolve in a volumetric flask, because the narrow neck makes it very hard to stir / crush the solid. In addition, you can’t heat a volumetric flask if the solid proves hard to dissolve, because heating will affect the shape and volume of the flask. Initially dissolving the solid in a beaker avoids these problems.
10. Rinse the glass rod with distilled water, making sure all the washings go into the solution in the beaker.
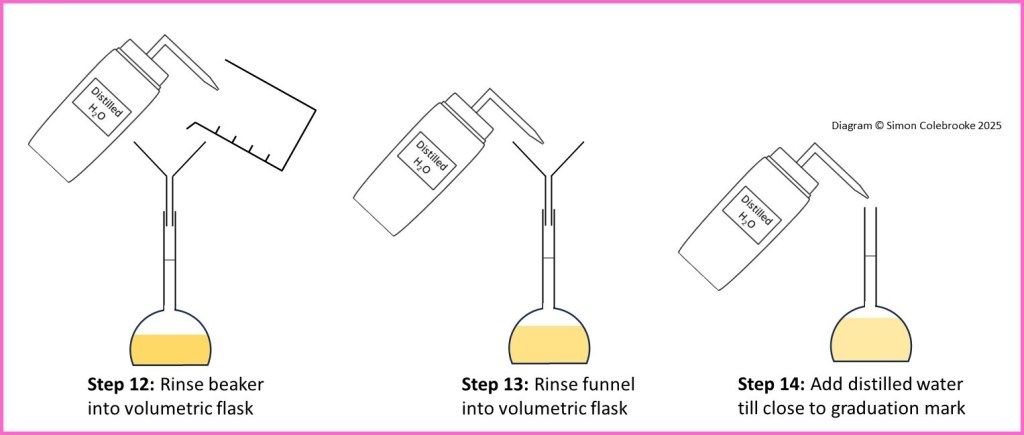
11. Pour the solution through a funnel (without filter paper) into the volumetric flask.

12. Rinse the beaker with distilled water and pour the washings through the funnel into the volumetric flask. Do this at least twice.
13. Rinse the funnel into the volumetric flask, using more distilled water and then remove it from the top of the flask.
WHY?
To be able to know the concentration of the standard solution it is essential that all of the solute weighed out at the start and dissolved in the beaker is transferred into the volumetric flask. Any loss of material will mean you don’t know how much solute there is in the flask at the end and so it will be impossible to determine the concentration.
14. Add distilled water to the volumetric flask until the solution starts to fill the thin neck of the flask and is about a cm below the graduation mark.
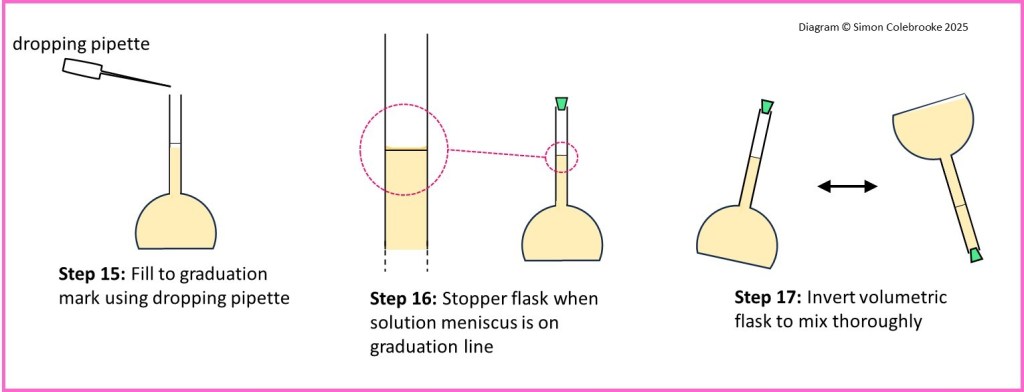
15. Use a dropping pipette to add more distilled water, keeping your eye level with the graduation line on the flask. Add the water dropwise until the bottom of the curved meniscus sits on the line.
16. Add a stopper to the volumetric flask.
17. Hold the stopper in place and invert (tip upside down) the flask several times to ensure it is well mixed.
WHY?
The density of the distilled water added near the end of the process will probably be less than that of the solution transferred from the beaker. Hence the added water will effectively float on top of the denser solution, meaning the concentration will be different at different places in the flask. Inverting the flask mixes the entire contents of the flask and makes the concentration the same throughout the whole solution.
18. Label the flask to show which solution in contains and the concentration.
Conclusion
At the start of this article three key points were described that are essential for ensuring the concentration of the standard solution is known. Having outlined the method it is now possible to see how they are achieved:
A). Accurately measure how much solid solute is used to make the solution.
The mass of the solid is determined by difference measurements, which take account of the minute traces of solid left on the weighing boat.
B). Make sure all of that solute ends up in the volumetric flask.
All the equipment which comes into contact with the solution being prepared is rinsed into the volumetric flask 0 this includes the glass rod, beaker and funnel.
C). Know exactly what volume of solution is prepared.
A high precision volumetric flask is used to measure the final volume of solution (the beaker with a poor scale is only used as a container). The flask is correctly filled using a dropping pipette until the meniscus sits on the graduation line.
Next steps:
To check your understanding of this section you can read the page on common errors made in this procedure and their effect on the solution concentration.
You can also read the article giving an example calculation for the preparation of a standard solution of sodium sulphate.
“Copyright Simon Colebrooke 1st November 2025″
Update History
Sub-headings and introductory sentences added 1st November 2025.
 chemistryexplained.uk
chemistryexplained.uk